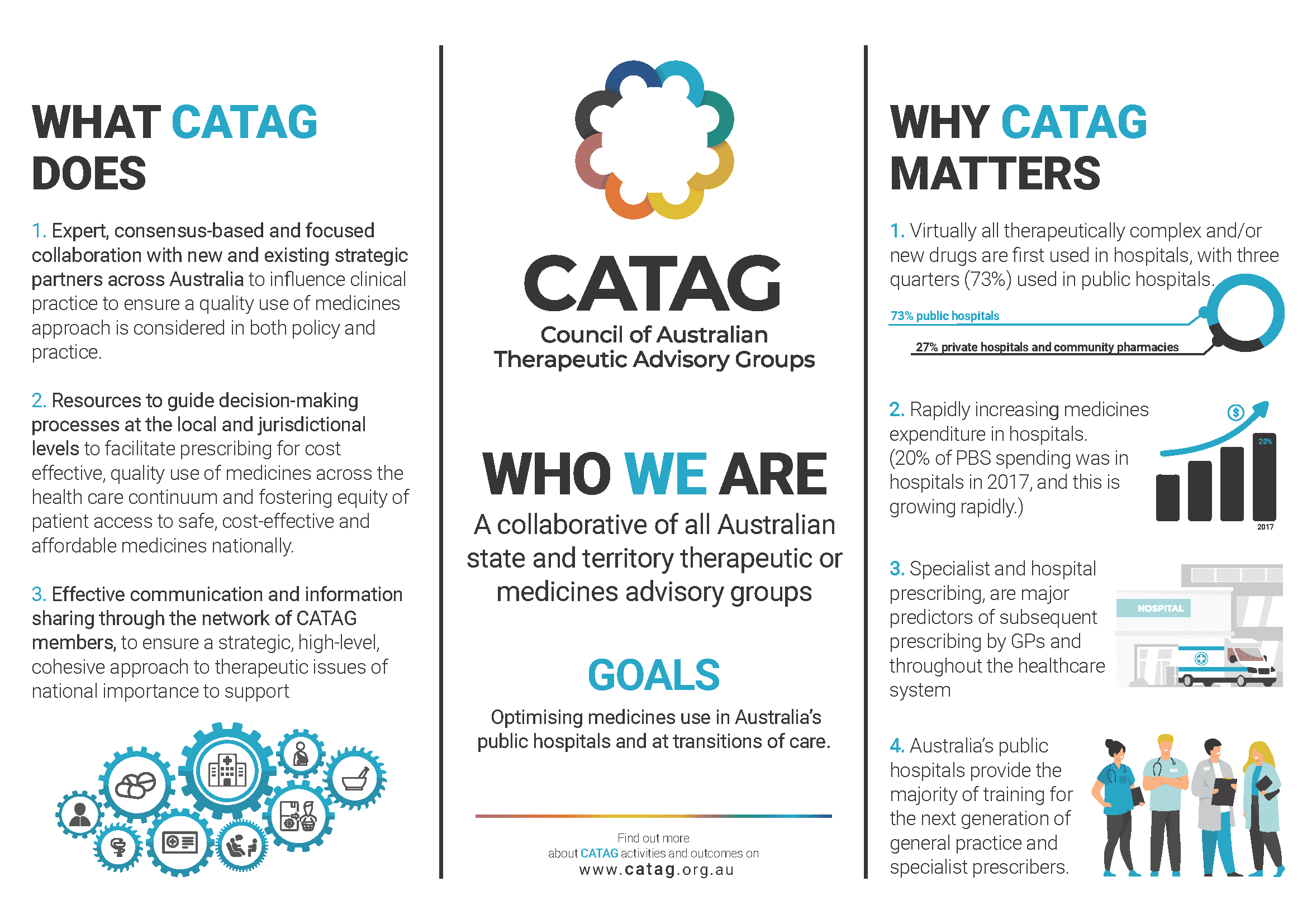
Established in 2005, CATAG is the peak national advisory body, a collaborative of all Australian state and territory therapeutic or medicines advisory groups.
CATAG is a collaboration of 9 state and territory Therapeutic or Medicines Advisory Groups:
- Canberra Health Services
- NSW Clinical Excellence Commission (CEC)
- NSW Therapeutic Advisory Group (NSW TAG)
- Northern Territory Health Medicines and Therapeutics Committee (NTMTC)
- Queensland Health Medicines Advisory Committee (QHMAC)
- South Australian Medicines Advisory Committee (SAMAC)
- Tasmanian Medicines Access and Advisory Committee (TMAAC)
- Victorian Therapeutics Advisory Group (VicTAG)
- Western Australian Therapeutics Advisory Group (WATAG)
Our purpose
Supports Quality Use of Medicine (QUM) in the acute care sector
CATAG aims to improve medicines management and use within the framework of the National Medicines Policy as it applies to clinical practice in Australian hospitals, at the interfaces of care, within the public sector and wider community. It promotes the equitable, safe, cost-effective and quality use of medicines, with the objective of realising the best possible health outcomes for all Australians. This also contributes to improved value and sustainability in the health system.
Improves medicines management and use
CATAG aims to improve medicines management and use within the framework of the National Medicines Policy as it applies to clinical practice in Australian hospitals, at transitions of care, within the public sector and wider community. It promotes the equitable, safe, cost-effective and quality use of medicines, with the objective of realising the best possible health outcomes for all Australians. This also contributes to improved value and sustainability in the health system.
Supports Drug and Therapeutics Committees (DTC)
CATAG supports Drug and Therapeutics Committees (DTC) to be well functioning which leads to a strong QUM framework within the individual health service. The framework then influences GP prescribing, through specialist prescribing practices filtering into the community when patients are discharged from the acute care setting.
Independent and cognizant of QUM issues and cost pressures
CATAG is independent and cognizant of QUM issues and the cost pressures faced by governments, policy decision makers, individual healthcare organisations and clinicians. CATAG is able to react immediately to changes in practice/evidence, with a particular focus on acute care.
Governance
CATAG is an independent entity, with current funding provided by the Australian Commission on Safety and Quality in Health Care and hosted by NSW Therapeutic Advisory Group (TAG). CATAG is governed by the CATAG Executive, which consists of the current Chair, the immediate past Chair and a representative from the hosting organisation (NSW TAG). The Executive is responsible for ensuring CATAG meets its functions and responsibilities.
Terms of reference
Our team
CATAG’s staff is dedicated to working towards facilitating quality use of medicines in Australian public hospitals.

Anita Shutt
Anita Shutt is the Director of Medication Strategy and Reform in the Department of Health, Tasmania. Anita is an ANZCAP recognised consultant pharmacist in the specialty fields of Leadership & Management, and Medicines Management. Anita maintains a passion for quality use of medicines, with a focus on building systems to improve medication safety and optimising healthcare through appropriate, sustainable and judicious use of medicines.
Anita has been a long-standing member of the Council of Australian Therapeutic Advisory Groups, acting as the Tasmanian jurisdictional representative for over a decade. More locally, Anita is a member of a number of state-wide clinical governance groups focussing on quality use of medicines, including the Tasmanian Medicines Access and Advisory Committee and the Tasmanian Medicines Safety Steering Committee.

Associate Professor Bhavini Patel
Bhavini Patel is the Executive Director of Medicines Management and Research for NT Health and has been the NT Executive COVID-19 Vaccine Lead since late 2020. Bhavini is an Advanced Practice Pharmacist with clinical expertise in renal and cardiovascular medicine and a special interest in how clinical pharmacists can improve the safety of and optimise outcomes associated with the quality use of medicines. Bhavini’s research interests include how to redesign systems of care to improve equity and access and how information technology can assist in this process.
Bhavini is an inaugural member of Council of Australian Therapeutic Advisory Groups and has held a number of leadership positions during her career. She has lived and worked in the NT for the last 20 years and finds the relaxed outdoor lifestyle a good balance to the demands of her work. Bhavini was the recipient of the Society of Hospital Pharmacists of Australia in 2011 Medal of Merit for establishing and growing hospital pharmacy practice in regional and remote Australia and was awarded Professional Excellence status in the NT for her Clinical Leadership practice in 2020.

Lisa Pulver
Lisa is an experienced pharmacist with an extensive range of experience within pharmacy, both in community and hospital, research, project management and training and education. Previously Lisa worked in hospital pharmacy at Royal Prince Alfred Hospital, Sydney and the Mater Misericordiae Hospitals, Brisbane where she was Team leader for Pharmacy services to the Children Hospital. She has had extensive involvement in the development and delivery of training and education to undergraduate students (nursing and pharmacy), pharmacists and other health care professionals.
Lisa has held academic appointments at the School of Pharmacy, The University of Sydney and at The University of Queensland, where she coordinated the Postgraduate Clinical Pharmacy Program. Lisa has published and presented at many conferences both in Australia and internationally.
Lisa has worked with the Council of Australian Therapeutic Advisory Groups since 2012 developing National Guiding Principles to support good medicines governance in hospitals.

Julie Briggs
Julie is a pharmacist whose career has focused on improving and implementing effective quality use of medicines. Her experience ranges from community to hospital pharmacy and includes time as an accredited pharmacist, a qualification she maintains to this day.
In recent years, Julie has turned towards improving medication management through intervention design, project management, and health promotion and education. A highlight of Julie’s career was working as a Program Officer at NPS MedicineWise where she led the design and development of quality use of medicine resources for Remote Area Aboriginal Health Services.
Funding
CATAG is funded by Australian Commission on Safety and Quality in Health Care together with in kind resources provided by the CATAG member organisations.
CATAG may also receive additional funding to undertake specific projects or participate in consortium led applications for grants.
